
Caesium, atomic structure Stock Image C023/2561 Science Photo Library
Cesium Electronic configuration. Electronic configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 6s 1 >> Back to key information about the elementBack to key information about the element

How To Find an Valence Cesium Electron Configuration (Cs)
Cesium is silvery-gold, soft, ductile alkali metal. It is liquid in a warm room, melting at 28.4 o C (83.1 o F). Cesium is one of the few metals that is liquid near room temperature. The others are gallium, francium and mercury. Cesium is an extremely reactive metal and the most alkaline of the elements.
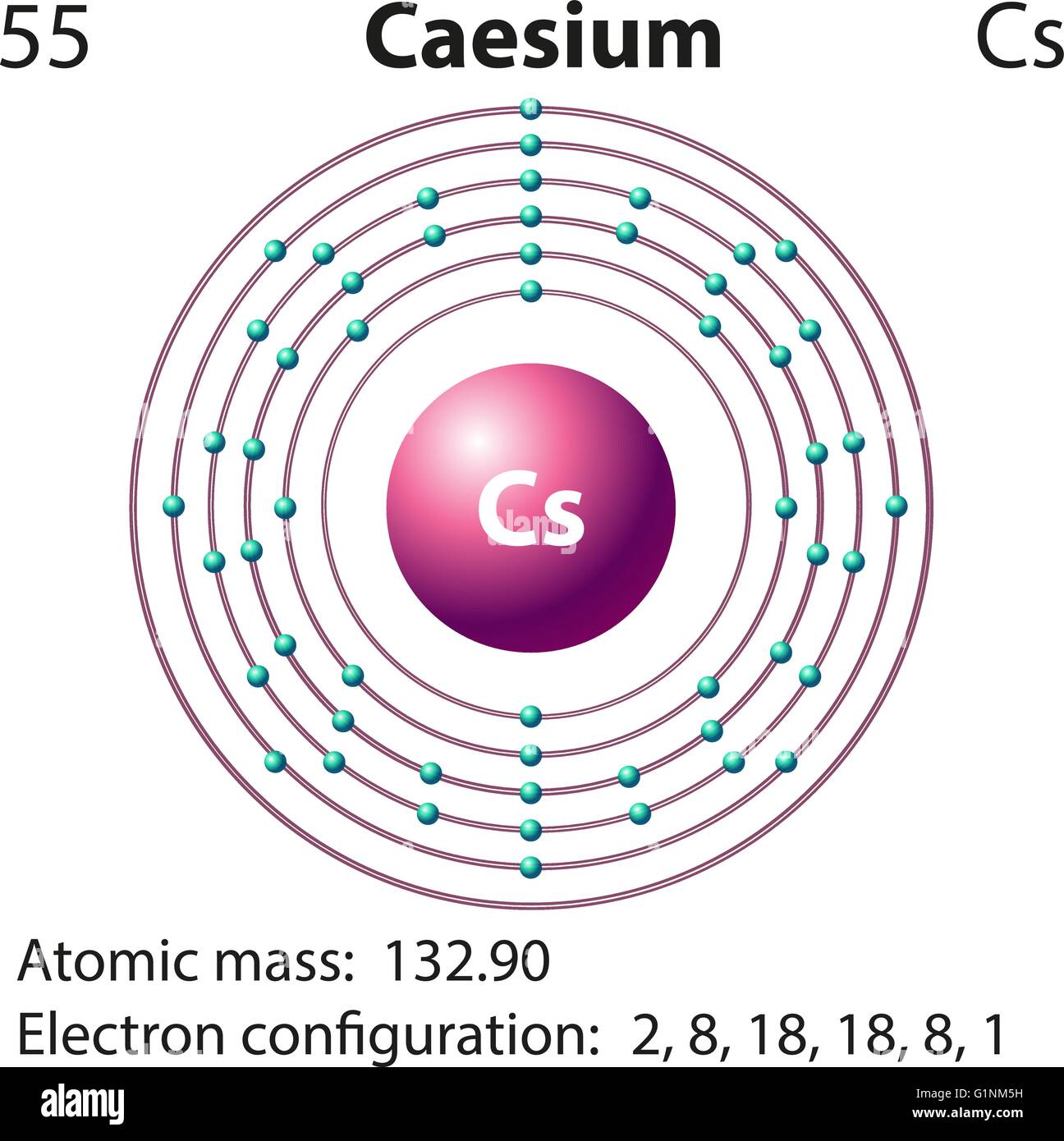
Symbol and electron diagram for Caesium illustration Stock Vector Image
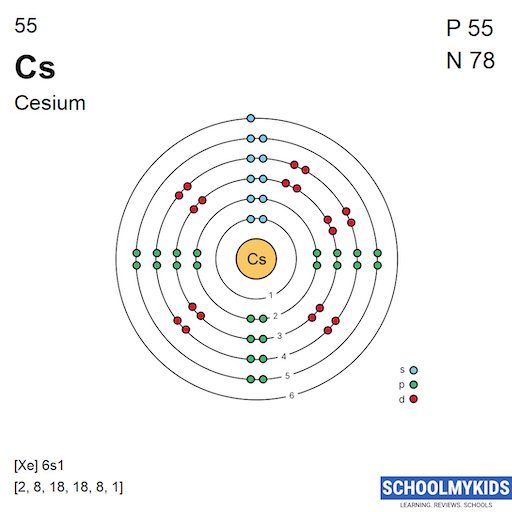
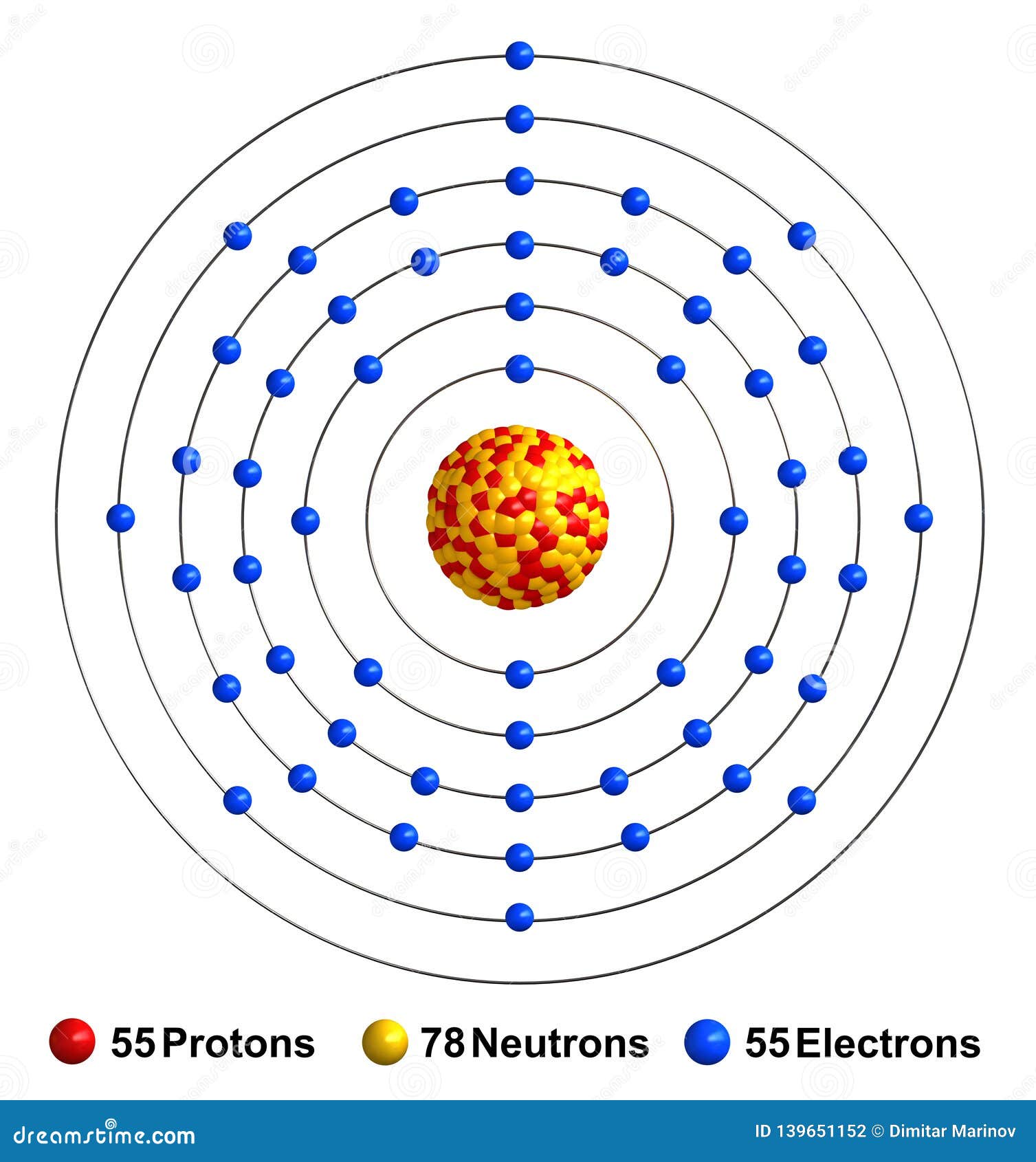
Caesium is a chemical element with atomic number 55 which means there are 55 protons and 55 electrons in the atomic structure. The chemical symbol for Caesium is Cs. Electron Configuration and Oxidation States of Caesium Electron configuration of Caesium is [Xe] 6s1. Possible oxidation states are +1. Electron Configuration

Cesium(Cs) electron configuration and orbital diagram
Caesium Electron Configuration Caesium has the occurrence form from the mining of pollucite and also has some other minor forms of occurrence. The chemical element was first discovered by German scientists in the year of 1860. It's quite an old chemical variant in itself that is useful for a number of practical domains.
Cs Cesium Element Information Facts, Properties, Trends, Uses and
Every element has its own Cesium Electron Configuration whereas here the electronic configuration for the element Cs is written as [Xe] 6 s1. The melting point of cesium is 83.3 o F and if we convert it to Celsius form, then it will be written as 28.5o C among the five elemental metals, cesium is one of the elements which are at room temperature.
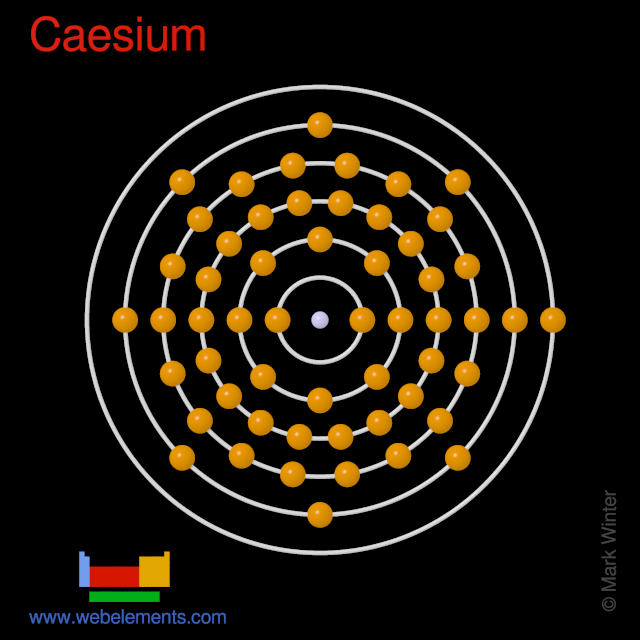
WebElements Periodic Table » Caesium » properties of free atoms
Electrons and Electron Configuration The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Caesium is 55.

Electron Configuration of Cesium Cs Lesson YouTube
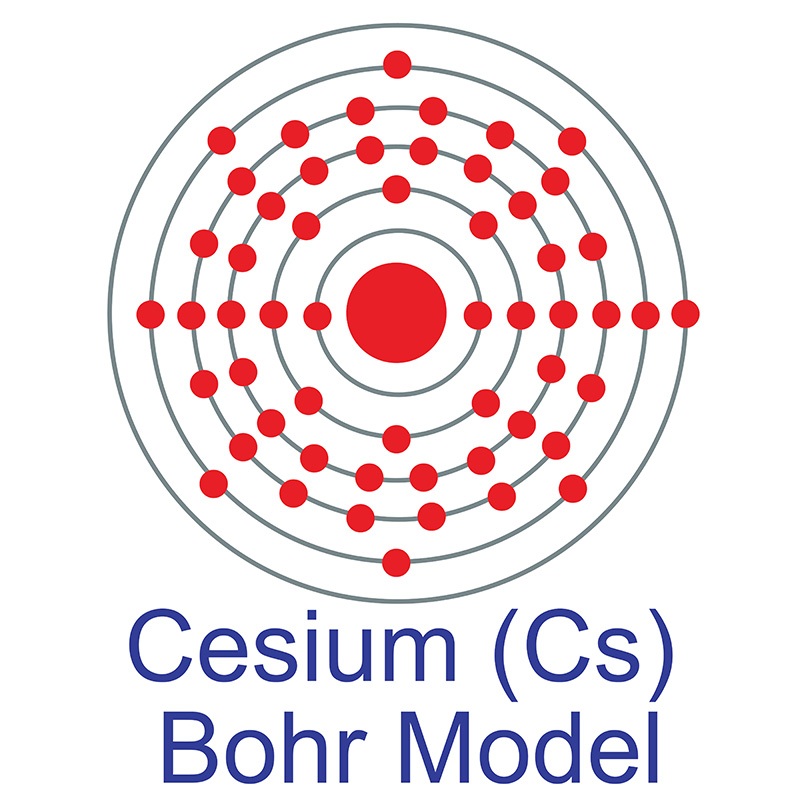
Caesium -. Cs: properties of free atoms. Caesium atoms have 55 electrons and the shell structure is 2.8.18.18.8.1. The ground state electron configuration of ground state gaseous neutral caesium is [ Xe ]. 6s1 and the term symbol is 2S1/2.
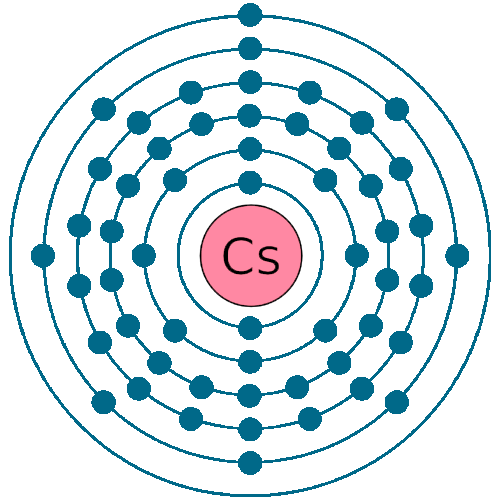
Cesium Cs (Elements 55) of Periodic Table Elements FlashCards
The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number between 0 and 14. Thus in the building-up process for the lanthanoids.
Cesium Atomic Orbital Diagram
Full electron configuration of cesium: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s1 xenon ← cesium → barium Cesium, complete electron configuration.

Symbol and electron diagram of Caesium Stock Vector Image & Art Alamy
0:00 / 2:59 How to write the electron configuration for Cesium (Cs and Cs+) Wayne Breslyn 723K subscribers Subscribe 29K views 3 years ago A step-by-step description of how to write the electron.

Caesium, atomic structure Stock Image C045/6398 Science Photo Library
Electron configuration for Cesium (element 55). Orbital diagram Cs (Cesium) is an element with position number in the periodic table. Located in the : 28.4 ℃. Electronic configuration of the Cesium atom in ascending order of orbital energies: Electronic configuration of the Cesium atom. Valence electrons. Orbital diagram

Cesium Electron configuration Symbol Atomic Number Atomic Mass
What is the electron configuration of cesium? The total number of electrons in cesium is fifty-five. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in cesium in specific rules in different orbits and orbitals is called the electron configuration of cesium.
Caesium stock illustration. Illustration of educational 139651152
The Electron: Crash Course Chemistry #5. Video 3.1.2 3.1. 2: An overview of the role of orbitals in electron configurations and how to write electron configurations. The relative energy of the subshells determine the order in which atomic orbitals are filled (1 s, 2 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, and so on).

Caesium, atomic structure Stock Image C013/1605 Science Photo Library
Electron configurations can be predicted by the position of an atom on the periodic table.. For example, take the elements in the first column of the periodic table: H, Li, Na, K, Rb, and Cs. Their electron configurations (abbreviated for the larger atoms) are as follows, with the valence shell electron configuration highlighted: Electrons.

WebElements Periodic Table » Caesium » properties of free atoms
Electron Configuration For Cesium: Caesium ( Caesium is the IUPAC spelling) or cesium (Cesium is the American spelling) is a chemical element which has a chemical symbol Cs. The atomic number of cesium is 55. It is a silvery-gold soft, alkali metal that has a melting point of 83.3 °F ( 28.5 °C), that makes it one of only five elemental metals which are liquid near or at […]

Electron Shell Diagrams of the 118 Elements
Electron Configuration Chart of All Elements (Full Chart) March 23, 2023 by Jay Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Free Gift for you: Interactive Periodic Table